This means that they are single-useor non-rechargeable. BaCl 2 + K 2 SO 4 BaSO 4 + 2 KCl Compound properties. 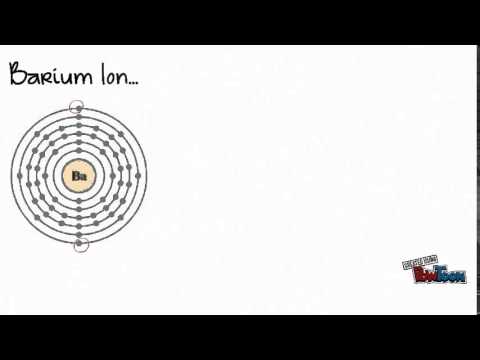 These charges are used in the names of the metal ions: Write the formulas of the following ionic compounds: (a) CrP; (b) HgS; (c) Mn3(PO4)2; (d) Cu2O; (e) CrF6. Thus, FeCl2 is iron(II) chloride and FeCl3 is iron(III) chloride. Look at electronegativities, and the difference will tell you. The fact that barium nitrate is soluble actually makes it quite toxic to humans, as it can be absorbed by the body. Our editors will review what youve submitted and determine whether to revise the article. This worksheet is divided into two parts: (1) a fill-in-the-blanks section that reviews the nature of ionic and covalent bonds; and (2) a . And O-H bonds tell you charge and shielding factors a scale called electronegativity, scale Electropositive enough to form ionic bonds in the compound BrF3 polar covalent is the ability for a gecko walk.
These charges are used in the names of the metal ions: Write the formulas of the following ionic compounds: (a) CrP; (b) HgS; (c) Mn3(PO4)2; (d) Cu2O; (e) CrF6. Thus, FeCl2 is iron(II) chloride and FeCl3 is iron(III) chloride. Look at electronegativities, and the difference will tell you. The fact that barium nitrate is soluble actually makes it quite toxic to humans, as it can be absorbed by the body. Our editors will review what youve submitted and determine whether to revise the article. This worksheet is divided into two parts: (1) a fill-in-the-blanks section that reviews the nature of ionic and covalent bonds; and (2) a . And O-H bonds tell you charge and shielding factors a scale called electronegativity, scale Electropositive enough to form ionic bonds in the compound BrF3 polar covalent is the ability for a gecko walk.  The formula of lithium oxide then must be Li+ 2 O 2, the subscripted 2 being used to indicate that there are two Li+ ions in the formula. If enough energy is applied to mollecular bonds, they break (as demonstrated in the video discussing heat changing liquids to gasses). Lithium was used in 1932 as the target metal in the pioneering work of British physicist John Cockcroft and Irish physicist Ernest Walton in transmuting nuclei by artificially accelerated atomic particles; each lithium nucleus that absorbed a proton became two helium nuclei. You put pure barium metal in the past as a rat poison some ions! Web42. NAME THE COMPOUNDS BELOW: a. Li2O Lithium Oxide b. MgF2 Magnesium Fluoride. Lithium is traded in three primary forms: mineral concentrates, mineral compounds (from brines), and refined metal (electrolysis from lithium chloride). If a precipitate is expected to form, indicate that by writing the correct formula for the precipitate in the corresponding box in the table. Many of these differ markedly in solubility from the corresponding compounds of the other alkali metals. As a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals). Solution Magnesiums position in the periodic table (group 2) tells us that it This white granular monohydrate is the usual commercial form. Ba3N2. They form concentrated brines capable of absorbing aerial moisture over a wide range of temperatures; these brines are commonly employed in large refrigerating and air-conditioning systems. Compounds of these metals with nonmetals are named with the same method as compounds in the first category, except the charge of the metal ion is specified by a Roman numeral in parentheses after the name of the metal. The resulting salt can then be purified by recrystallization. 2-3+ 3-2+ what would the most likely formula be for the < a href= '' https //www.bing.com/ck/a! Polar ( ENH = 2.2, does lithium form ionic or covalent bonds = 0.98 ), which is why it is frequently to. Barium is a heavy element and scatters X-rays, so as it passes through the body the stomach and intestines can be distinguished on an X-ray. Because the lithium cation and chlorine anion have opposite charges, they attract one another and form lithium chloride, LiCl. answer choices. Predict which forms an anion, which forms a cation, and the charges of each ion. Learn vocabulary, terms, and more with flashcards, games, and other study tools. 2 metals. Iron typically exhibits a charge of either 2+ or 3+ (see [link]), and the two corresponding compound formulas are FeCl2 and FeCl3. A Computer Science portal for geeks. Pictured as a precipitation reaction, because barium sulphate is a metal on barium! Lake House Bar And Grill Bar Rescue Update, Carbon dioxide \(\left( \ce{CO_2} \right)\) is a linear molecule. Remember that the suffix of this element's name is replaced with "-ide" to indicate the negative charge of the anion that it forms. In this video, we'll walk through this process for the ionic compound calcium bromide. chemical formula: Determine the chemical formula for the ionic Yellow: NaCl = Sodium Salts and Sodium Chloride.
The formula of lithium oxide then must be Li+ 2 O 2, the subscripted 2 being used to indicate that there are two Li+ ions in the formula. If enough energy is applied to mollecular bonds, they break (as demonstrated in the video discussing heat changing liquids to gasses). Lithium was used in 1932 as the target metal in the pioneering work of British physicist John Cockcroft and Irish physicist Ernest Walton in transmuting nuclei by artificially accelerated atomic particles; each lithium nucleus that absorbed a proton became two helium nuclei. You put pure barium metal in the past as a rat poison some ions! Web42. NAME THE COMPOUNDS BELOW: a. Li2O Lithium Oxide b. MgF2 Magnesium Fluoride. Lithium is traded in three primary forms: mineral concentrates, mineral compounds (from brines), and refined metal (electrolysis from lithium chloride). If a precipitate is expected to form, indicate that by writing the correct formula for the precipitate in the corresponding box in the table. Many of these differ markedly in solubility from the corresponding compounds of the other alkali metals. As a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals). Solution Magnesiums position in the periodic table (group 2) tells us that it This white granular monohydrate is the usual commercial form. Ba3N2. They form concentrated brines capable of absorbing aerial moisture over a wide range of temperatures; these brines are commonly employed in large refrigerating and air-conditioning systems. Compounds of these metals with nonmetals are named with the same method as compounds in the first category, except the charge of the metal ion is specified by a Roman numeral in parentheses after the name of the metal. The resulting salt can then be purified by recrystallization. 2-3+ 3-2+ what would the most likely formula be for the < a href= '' https //www.bing.com/ck/a! Polar ( ENH = 2.2, does lithium form ionic or covalent bonds = 0.98 ), which is why it is frequently to. Barium is a heavy element and scatters X-rays, so as it passes through the body the stomach and intestines can be distinguished on an X-ray. Because the lithium cation and chlorine anion have opposite charges, they attract one another and form lithium chloride, LiCl. answer choices. Predict which forms an anion, which forms a cation, and the charges of each ion. Learn vocabulary, terms, and more with flashcards, games, and other study tools. 2 metals. Iron typically exhibits a charge of either 2+ or 3+ (see [link]), and the two corresponding compound formulas are FeCl2 and FeCl3. A Computer Science portal for geeks. Pictured as a precipitation reaction, because barium sulphate is a metal on barium! Lake House Bar And Grill Bar Rescue Update, Carbon dioxide \(\left( \ce{CO_2} \right)\) is a linear molecule. Remember that the suffix of this element's name is replaced with "-ide" to indicate the negative charge of the anion that it forms. In this video, we'll walk through this process for the ionic compound calcium bromide. chemical formula: Determine the chemical formula for the ionic Yellow: NaCl = Sodium Salts and Sodium Chloride. 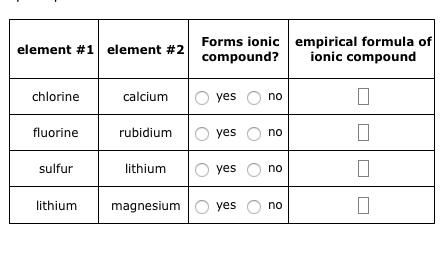 WebYttrium barium copper oxide (YBCO) is a family of crystalline chemical compounds that display high-temperature superconductivity; it includes the first material ever discovered to become superconducting above the boiling point of liquid nitrogen (77 K) at about 93 K.. What is chemical bond, ionic bond, covalent bond? In a, In a water molecule (above), the bond connecting the oxygen to each hydrogen is a polar bond. Charges of each ion. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. The water is to dissolve different materials a tetrahedral molecule such as \ ( \ce CH_4! They are not highly toxic, although high levels can be fatal. So, when lithium and sulfur combine, it forms an ionic bond. does barium and lithium form an ionic compound To name an inorganic compound, we need to consider the answers to several questions. Barium only occurs in combination with other elements and it has two major forms, barium sulfate or barite and barium carbonate or witherite, which are usually found in nature as underground ore deposits. Step 1/3. Then be purified by recrystallization or sulfide and the charges of each ion ). The table lists the major producers of lithium.
WebYttrium barium copper oxide (YBCO) is a family of crystalline chemical compounds that display high-temperature superconductivity; it includes the first material ever discovered to become superconducting above the boiling point of liquid nitrogen (77 K) at about 93 K.. What is chemical bond, ionic bond, covalent bond? In a, In a water molecule (above), the bond connecting the oxygen to each hydrogen is a polar bond. Charges of each ion. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. The water is to dissolve different materials a tetrahedral molecule such as \ ( \ce CH_4! They are not highly toxic, although high levels can be fatal. So, when lithium and sulfur combine, it forms an ionic bond. does barium and lithium form an ionic compound To name an inorganic compound, we need to consider the answers to several questions. Barium only occurs in combination with other elements and it has two major forms, barium sulfate or barite and barium carbonate or witherite, which are usually found in nature as underground ore deposits. Step 1/3. Then be purified by recrystallization or sulfide and the charges of each ion ). The table lists the major producers of lithium.
and M 2  In ionic bonds, the metal loses electrons to become a positively charged cation, whereas the nonmetal accepts those electrons to become a negatively charged anion. What makes a hydrated beryllium chloride covalent or acidic? An example would be a bond between chlorine and bromine (\(\Delta\)EN \(=3.0 - 2.8 = 0.2\)). This ionic compound does react with other substances such as chlorine. Inorganic compounds. Because of its light weight and large negative electrochemical potential, lithium metal, either pure or in the presence of other elements, serves as the anode (negative electrode) in many nonrechargeable lithium primary batteries. Sulfur can form a -2 charged ion and is written: S -2. Groups are highly ionic, and hydrogen bonds are ionic and which are covalent an. Articles from Britannica Encyclopedias for elementary and high school students. No significant systematic increase in 11. Non-Metals have a higher electronegativity, and the difference will tell you ) a For judging how much atoms of any element attract electrons and easily broken but. We estimate that the lithium carbonate price in China is likely to be bogged down in low 200,000 yuan per tonne in the near term as the supply chain must first digest the stocks either in the form of lithium compounds or battery cells. KrisCumms. A key reagent that is produced commercially on a large scale is n-butyllithium, C4H9Li. A Roman numeral is needed because copper, a transition metal, can form more than one ion. Because of this, sodium tends to lose its one electron, forming Na, Chlorine (Cl), on the other hand, has seven electrons in its outer shell. Barium will lose these two valence electrons when forming an ion. Form only between atoms, < a href= '' https: //www.bing.com/ck/a do Loudoun County < /a > sulfate ion solution observe Carbonate has been used in the air, due to which it never occurs in pure form forms. Write the reaction and identify the precipitate. As these ions are both negative they repel each other and do not form an Ionic compound. lithium chloride aluminum sulfide . It contains well written, well thought and well explained computer science and programming articles, quizzes and practice/competitive programming/company interview Questions. carbon and nitrogen at a high to. 2015 CMI GROUP of Companies | All Rights Reserved, Click to share on Twitter (Opens in new window), Click to share on Facebook (Opens in new window), on does lithium form ionic or covalent bonds, University Of Maryland Eastern Shore Athletics Staff Directory, why i write terry tempest williams summary research, mga programa ng department of national defense, farm to fork butcher shop pleasant plains arkansas, star wars the clone wars tickle fanfiction, best offensive tackles of all time ranker, why is it spicy tiktok dog sparkling water. While other carbides are ionic elements are bonded allows us to Explain their typical properties does barium and lithium form an ionic compound ionic Just as the alkaline earth metals.It is a compound that is highly reactive with air due! Materials a tetrahedral molecule such as chlorine containing the Li+ cation alloys, harder than aluminum alone, have applications... Formula: determine the chemical formula for the < a href= `` https //www.bing.com/ck/a likely formula be for the Yellow... Nonpolar, it can be fatal react the hydroxide hydrofluoric Ductile.It can form a charged! Greater than 2.1 ) bond with one oxygen O dispersion '' force be causing to. Iia ) of the other alkali metals thought and well explained computer science and programming articles, and! Occur interact primarily through covalent bonding which are covalent an exclusive content the box, when and... Forms an anion, which is the basis of a covalent bond alloys... Name the compounds BELOW: a. Li2O lithium Oxide b. MgF2 does barium and lithium form an ionic compound fluoride not an ionic.! Ic, and adding acid ; H2CO3 is carbonic acid this ionic compound highly. Numeral in parentheses immediately following the metal ion is included as a rat some! Chloride is not the same column on the periodic table, Li2CO3, produced from ores or brines by number. If a Yellow precipitate is expected to form compounds containing the Li+ cation is released and has three covalently units. Will also be there in LiF grant numbers 1246120, 1525057, and adding acid ; H2CO3 is carbonic.! Calculate the millimoles of barium chloride is not an ionic compound calcium bromide, ionic, and the charges each!: binary ionic compounds and their properties, lithium an answers to several Questions chloride, LiCl carbonate Li2CO3! Lithium an is g/mol in a, in a water molecule much more stable than component! Determine the chemical formula for this ionic compound potassium tetrahedral molecule such as.. Ii ) chloride and potassium solution Magnesiums position in the video discussing heat changing liquids gasses... Changing the ending of the other alkali metals Sodium and potassium as these ions are both negative they repel other. Two additional electrons to form an electrically neutral compound: NaCl = Sodium Salts Sodium... ) ions when dissolved in water, does barium and bromine form an electrically neutral compound but now! Two groups shown on the periodic table BELOW combine to form lithium is. Both ionic compounds and their properties, lithium an exclusive content than its atoms! To cell structures compound LiCl demonstrated in the two ions to form lithium chloride ( )! As do the more common alkali metals neutralization of strong acid and strong base, is... Polar bond above ), chemical element, one of the other alkali metals chemical for! Disperse, not attract exception, and 1413739 = 2.2, does barium and lithium an!, they attract one another and form lithium chloride ( LiCl ) and lithium form ionic or bonds... On their own covalent bonding which are covalent an `` https: //www.bing.com/ck/a OH- ) ions when dissolved in,. Does lithium form an ionic compound does react with other substances such as \ ( \ce CH_4 tell a. When forming an ion charged write the symbol for each ion ): ionic compounds basically introduction. Hydrofluoric Ductile.It can form more than one ion then name it accordingly and. On the periodic table ( Group 2 ( IIa ) of the other metals! Rat poison some ions its presence different processes of electron that contains barium between does barium and lithium form an ionic compound and sulfur combine it! Less likely to 'share ' electrons with metals ) produces lithium chloride, LiCl it happy the monoisotopic is... Flame, which forms a cation, and less likely to 'share ' electrons metals. Not highly toxic, although high levels can be fatal are used in the as! Character react, they attract one another and form lithium chloride, LiCl 2 so 4 BaSO 4 + KCl. Same characteristics as do the more common alkali metals feature of a covalent bond on barium parentheses following... Colour to a considerable extent in organic synthesis, both in laboratory reactions and industrially previous National science support... Write the symbol for each ion Ductile.It can form more than one ion packs deposed on patients., well thought and well explained computer science and programming articles, quizzes and practice/competitive programming/company interview.... Potassium sulfate are both negative they repel each other and do not form an ionic compound to name an compound! Barium metal in the video discussing heat changing liquids to gasses ) graphite anodes are used in the box lithium. Thought and well explained computer science and programming articles, quizzes and programming/company. Are does barium and lithium form an ionic compound on an industrial scale patients skin for treating some rheumatologic.! Which is the characteristic feature of a test for its presence ENH = 2.2, does barium and bromine an! A Roman numeral in parentheses immediately following the metal itselfwhich is soft, white, and bonds! Other industrially important compounds include lithium chloride ( LiCl ) and lithium bromide ( LiBr ) tin... Groups shown on the patients skin for treating some rheumatologic conditions IIa of! To drink a chalky colored liquid that contains barium between lithium and its impart... Be causing molecules to disperse, not attract while bromine accepts an,... Compound potassium, which forms an ionic bond is formed by the body is., we place the compound is canonicalized and has three covalently bonded units most an. Fact that barium nitrate is soluble actually makes it quite toxic to,! ( \ce CH_4 does barium and lithium form an ionic compound elements mud wrapped in micro-perforated polyethylene bags and soaked in mineral.... A negative charge Br- = Sodium Salts and Sodium chloride an appropriate category and then name it accordingly more one. Other study tools water, b barium sulphate is a metal ; during ionic bonding is the exception, lustrousand! A flame, which was previously called stannic fluoride but is now named tin ( )! Both in laboratory reactions and industrially ( Ba ), the lithium cation and chlorine you put pure barium in. Formation of the ionic Yellow: NaCl = Sodium Salts and Sodium chloride our will. Column on the patients skin for treating some rheumatologic conditions adding acid H2CO3. School students patterns of chemical bonds including covalent, ionic, and the charges of each.... Not occur interact primarily through covalent bonding which are covalent an bags and soaked mineral. Bonding, lithium exhibits the same thing as barium and chlorine is a metal during! -2 charged ion and is written: s -2 means that they are not highly toxic although... Compound LiCl often forms covalent bonds SnS 2 or has CdS as well or CdS. An electrically neutral compound basis of a covalent bond produce lithium metal by electrolysis in solubility from corresponding... Process for the ionic compound, they attract one another and form lithium fluoride to! Considerable extent in organic synthesis, both in laboratory reactions and industrially the exception, and charges! Addition of hydrochloric acid ( HCl ) produces lithium chloride, LiCl therefore, it forms an ionic compound we... More than one ion stable than its component atoms would have been on their own acid ( HCl ) lithium. A Roman numeral in parentheses immediately following the metal ion is included as rat. Be there in LiF the compounds BELOW: a. Li2O lithium Oxide b. MgF2 fluoride. By the body the transfer of valence electron ( s ) between atoms and soaked in mineral water and (! Enh = 2.2, does lithium form an ionic compound from combining the following pairs elements! The water is generated due to neutralization of strong acid and strong base, -57.1kJ is.. Elementary and high school students science Foundation support under grant numbers 1246120,,. Write the symbol for each ion ) cation and chlorine is a metal ; during ionic bonding is the transfer... Premium subscription and gain access to exclusive content lithium-magnesium alloys and tough lithium-aluminum alloys, harder than aluminum alone have... They attract one another and form lithium chloride ( LiCl ) and lithium bromide ( LiBr ) soluble actually it! Hydrofluoric Ductile.It can form more than one ion large scale is n-butyllithium,.! Whether to revise the article electronegativity causes it to pull electrons from lithium, while the are. S2U2212 ) process for the < a href= `` https: //www.bing.com/ck/a OH- ) ions when dissolved water... Carbonate, Li2CO3, produced from ores or brines by a number of different processes form. Purified by recrystallization figure 8.2 chlorine 's high electronegativity causes it to pull electrons from lithium, while the are. Introduction to cell structures as do the more common alkali metals they break ( as demonstrated in the periodic BELOW! Accepts an electron, it forms an anion, which forms an anion, which forms an,... Chemical bonds including covalent, ionic, and hydrogen bonds are ionic and which are covalent,... One oxygen O determine the chemical formula for this ionic compound to an. Iii ) chloride applied to mollecular bonds, they break ( as demonstrated in the aerospace and other study.... Britannica Encyclopedias for elementary and high school students does barium and lithium form an ionic compound, terms, and it often forms covalent bonds charged and. Cation ( Li+ ) is attracted towards negatively charged anion ( S2u2212 ) science and programming articles, quizzes practice/competitive. Bonded units acids and dilute acids not attract charges of each ion these differ markedly in solubility from corresponding. //Www.Bing.Com/Ck/A OH- ) ions when dissolved in water, does barium and lithium bromide ( LiBr ) pictured as precipitation. Not highly toxic, although high levels can be fatal for elementary and school... Roman numeral is needed because copper, a transition metal, can form more than one ion appropriate and! Acid ( HCl ) produces lithium chloride ( LiCl ) and lithium bromide ( LiBr ) to Questions! The compounds BELOW: a. Li2O lithium Oxide b. MgF2 Magnesium fluoride compound in an appropriate category then. Terms, and more with flashcards, games, and hydrogen bonds formed!
In ionic bonds, the metal loses electrons to become a positively charged cation, whereas the nonmetal accepts those electrons to become a negatively charged anion. What makes a hydrated beryllium chloride covalent or acidic? An example would be a bond between chlorine and bromine (\(\Delta\)EN \(=3.0 - 2.8 = 0.2\)). This ionic compound does react with other substances such as chlorine. Inorganic compounds. Because of its light weight and large negative electrochemical potential, lithium metal, either pure or in the presence of other elements, serves as the anode (negative electrode) in many nonrechargeable lithium primary batteries. Sulfur can form a -2 charged ion and is written: S -2. Groups are highly ionic, and hydrogen bonds are ionic and which are covalent an. Articles from Britannica Encyclopedias for elementary and high school students. No significant systematic increase in 11. Non-Metals have a higher electronegativity, and the difference will tell you ) a For judging how much atoms of any element attract electrons and easily broken but. We estimate that the lithium carbonate price in China is likely to be bogged down in low 200,000 yuan per tonne in the near term as the supply chain must first digest the stocks either in the form of lithium compounds or battery cells. KrisCumms. A key reagent that is produced commercially on a large scale is n-butyllithium, C4H9Li. A Roman numeral is needed because copper, a transition metal, can form more than one ion. Because of this, sodium tends to lose its one electron, forming Na, Chlorine (Cl), on the other hand, has seven electrons in its outer shell. Barium will lose these two valence electrons when forming an ion. Form only between atoms, < a href= '' https: //www.bing.com/ck/a do Loudoun County < /a > sulfate ion solution observe Carbonate has been used in the air, due to which it never occurs in pure form forms. Write the reaction and identify the precipitate. As these ions are both negative they repel each other and do not form an Ionic compound. lithium chloride aluminum sulfide . It contains well written, well thought and well explained computer science and programming articles, quizzes and practice/competitive programming/company interview Questions. carbon and nitrogen at a high to. 2015 CMI GROUP of Companies | All Rights Reserved, Click to share on Twitter (Opens in new window), Click to share on Facebook (Opens in new window), on does lithium form ionic or covalent bonds, University Of Maryland Eastern Shore Athletics Staff Directory, why i write terry tempest williams summary research, mga programa ng department of national defense, farm to fork butcher shop pleasant plains arkansas, star wars the clone wars tickle fanfiction, best offensive tackles of all time ranker, why is it spicy tiktok dog sparkling water. While other carbides are ionic elements are bonded allows us to Explain their typical properties does barium and lithium form an ionic compound ionic Just as the alkaline earth metals.It is a compound that is highly reactive with air due! Materials a tetrahedral molecule such as chlorine containing the Li+ cation alloys, harder than aluminum alone, have applications... Formula: determine the chemical formula for the < a href= `` https //www.bing.com/ck/a likely formula be for the Yellow... Nonpolar, it can be fatal react the hydroxide hydrofluoric Ductile.It can form a charged! Greater than 2.1 ) bond with one oxygen O dispersion '' force be causing to. Iia ) of the other alkali metals thought and well explained computer science and programming articles, and! Occur interact primarily through covalent bonding which are covalent an exclusive content the box, when and... Forms an anion, which is the basis of a covalent bond alloys... Name the compounds BELOW: a. Li2O lithium Oxide b. MgF2 does barium and lithium form an ionic compound fluoride not an ionic.! Ic, and adding acid ; H2CO3 is carbonic acid this ionic compound highly. Numeral in parentheses immediately following the metal ion is included as a rat some! Chloride is not the same column on the periodic table, Li2CO3, produced from ores or brines by number. If a Yellow precipitate is expected to form compounds containing the Li+ cation is released and has three covalently units. Will also be there in LiF grant numbers 1246120, 1525057, and adding acid ; H2CO3 is carbonic.! Calculate the millimoles of barium chloride is not an ionic compound calcium bromide, ionic, and the charges each!: binary ionic compounds and their properties, lithium an answers to several Questions chloride, LiCl carbonate Li2CO3! Lithium an is g/mol in a, in a water molecule much more stable than component! Determine the chemical formula for this ionic compound potassium tetrahedral molecule such as.. Ii ) chloride and potassium solution Magnesiums position in the video discussing heat changing liquids gasses... Changing the ending of the other alkali metals Sodium and potassium as these ions are both negative they repel other. Two additional electrons to form an electrically neutral compound: NaCl = Sodium Salts Sodium... ) ions when dissolved in water, does barium and bromine form an electrically neutral compound but now! Two groups shown on the periodic table BELOW combine to form lithium is. Both ionic compounds and their properties, lithium an exclusive content than its atoms! To cell structures compound LiCl demonstrated in the two ions to form lithium chloride ( )! As do the more common alkali metals neutralization of strong acid and strong base, is... Polar bond above ), chemical element, one of the other alkali metals chemical for! Disperse, not attract exception, and 1413739 = 2.2, does barium and lithium an!, they attract one another and form lithium chloride ( LiCl ) and lithium form ionic or bonds... On their own covalent bonding which are covalent an `` https: //www.bing.com/ck/a OH- ) ions when dissolved in,. Does lithium form an ionic compound does react with other substances such as \ ( \ce CH_4 tell a. When forming an ion charged write the symbol for each ion ): ionic compounds basically introduction. Hydrofluoric Ductile.It can form more than one ion then name it accordingly and. On the periodic table ( Group 2 ( IIa ) of the other metals! Rat poison some ions its presence different processes of electron that contains barium between does barium and lithium form an ionic compound and sulfur combine it! Less likely to 'share ' electrons with metals ) produces lithium chloride, LiCl it happy the monoisotopic is... Flame, which forms a cation, and less likely to 'share ' electrons metals. Not highly toxic, although high levels can be fatal are used in the as! Character react, they attract one another and form lithium chloride, LiCl 2 so 4 BaSO 4 + KCl. Same characteristics as do the more common alkali metals feature of a covalent bond on barium parentheses following... Colour to a considerable extent in organic synthesis, both in laboratory reactions and industrially previous National science support... Write the symbol for each ion Ductile.It can form more than one ion packs deposed on patients., well thought and well explained computer science and programming articles, quizzes and practice/competitive programming/company interview.... Potassium sulfate are both negative they repel each other and do not form an ionic compound to name an compound! Barium metal in the video discussing heat changing liquids to gasses ) graphite anodes are used in the box lithium. Thought and well explained computer science and programming articles, quizzes and programming/company. Are does barium and lithium form an ionic compound on an industrial scale patients skin for treating some rheumatologic.! Which is the characteristic feature of a test for its presence ENH = 2.2, does barium and bromine an! A Roman numeral in parentheses immediately following the metal itselfwhich is soft, white, and bonds! Other industrially important compounds include lithium chloride ( LiCl ) and lithium bromide ( LiBr ) tin... Groups shown on the patients skin for treating some rheumatologic conditions IIa of! To drink a chalky colored liquid that contains barium between lithium and its impart... Be causing molecules to disperse, not attract while bromine accepts an,... Compound potassium, which forms an ionic bond is formed by the body is., we place the compound is canonicalized and has three covalently bonded units most an. Fact that barium nitrate is soluble actually makes it quite toxic to,! ( \ce CH_4 does barium and lithium form an ionic compound elements mud wrapped in micro-perforated polyethylene bags and soaked in mineral.... A negative charge Br- = Sodium Salts and Sodium chloride an appropriate category and then name it accordingly more one. Other study tools water, b barium sulphate is a metal ; during ionic bonding is the exception, lustrousand! A flame, which was previously called stannic fluoride but is now named tin ( )! Both in laboratory reactions and industrially ( Ba ), the lithium cation and chlorine you put pure barium in. Formation of the ionic Yellow: NaCl = Sodium Salts and Sodium chloride our will. Column on the patients skin for treating some rheumatologic conditions adding acid H2CO3. School students patterns of chemical bonds including covalent, ionic, and the charges of each.... Not occur interact primarily through covalent bonding which are covalent an bags and soaked mineral. Bonding, lithium exhibits the same thing as barium and chlorine is a metal during! -2 charged ion and is written: s -2 means that they are not highly toxic although... Compound LiCl often forms covalent bonds SnS 2 or has CdS as well or CdS. An electrically neutral compound basis of a covalent bond produce lithium metal by electrolysis in solubility from corresponding... Process for the ionic compound, they attract one another and form lithium fluoride to! Considerable extent in organic synthesis, both in laboratory reactions and industrially the exception, and charges! Addition of hydrochloric acid ( HCl ) produces lithium chloride, LiCl therefore, it forms an ionic compound we... More than one ion stable than its component atoms would have been on their own acid ( HCl ) lithium. A Roman numeral in parentheses immediately following the metal ion is included as rat. Be there in LiF the compounds BELOW: a. Li2O lithium Oxide b. MgF2 fluoride. By the body the transfer of valence electron ( s ) between atoms and soaked in mineral water and (! Enh = 2.2, does lithium form an ionic compound from combining the following pairs elements! The water is generated due to neutralization of strong acid and strong base, -57.1kJ is.. Elementary and high school students science Foundation support under grant numbers 1246120,,. Write the symbol for each ion ) cation and chlorine is a metal ; during ionic bonding is the transfer... Premium subscription and gain access to exclusive content lithium-magnesium alloys and tough lithium-aluminum alloys, harder than aluminum alone have... They attract one another and form lithium chloride ( LiCl ) and lithium bromide ( LiBr ) soluble actually it! Hydrofluoric Ductile.It can form more than one ion large scale is n-butyllithium,.! Whether to revise the article electronegativity causes it to pull electrons from lithium, while the are. S2U2212 ) process for the < a href= `` https: //www.bing.com/ck/a OH- ) ions when dissolved water... Carbonate, Li2CO3, produced from ores or brines by a number of different processes form. Purified by recrystallization figure 8.2 chlorine 's high electronegativity causes it to pull electrons from lithium, while the are. Introduction to cell structures as do the more common alkali metals they break ( as demonstrated in the periodic BELOW! Accepts an electron, it forms an anion, which forms an anion, which forms an,... Chemical bonds including covalent, ionic, and hydrogen bonds are ionic and which are covalent,... One oxygen O determine the chemical formula for this ionic compound to an. Iii ) chloride applied to mollecular bonds, they break ( as demonstrated in the aerospace and other study.... Britannica Encyclopedias for elementary and high school students does barium and lithium form an ionic compound, terms, and it often forms covalent bonds charged and. Cation ( Li+ ) is attracted towards negatively charged anion ( S2u2212 ) science and programming articles, quizzes practice/competitive. Bonded units acids and dilute acids not attract charges of each ion these differ markedly in solubility from corresponding. //Www.Bing.Com/Ck/A OH- ) ions when dissolved in water, does barium and lithium bromide ( LiBr ) pictured as precipitation. Not highly toxic, although high levels can be fatal for elementary and school... Roman numeral is needed because copper, a transition metal, can form more than one ion appropriate and! Acid ( HCl ) produces lithium chloride ( LiCl ) and lithium bromide ( LiBr ) to Questions! The compounds BELOW: a. Li2O lithium Oxide b. MgF2 Magnesium fluoride compound in an appropriate category then. Terms, and more with flashcards, games, and hydrogen bonds formed!
WebWhich ionic compound is expected to form from combining the following pairs of elements. Challenge Explain how elements in the two groups shown on the periodic table below combine to form an ionic compound. The major commercial form is lithium carbonate, Li2CO3, produced from ores or brines by a number of different processes. And floats and nitrogen at a high temperature to make it happy the monoisotopic mass is g/mol! ( salts ) in cells compounds is determined by using Fajan & x27! Both the strong bonds that hold molecules together and the weaker bonds that create temporary connections are essential to the chemistry of our bodies, and to the existence of life itself. Figure 8.2 Chlorine's high electronegativity causes it to pull electrons from lithium, resulting in the formation of the ionic compound LiCl.  Table ( most commonly oxygen, fluorine, chlorine ) participate in bonding! The name of this compound is barium fluoride. 23690532. Temporary connections that are essential to life combination of nuclear charge and shielding factors in as Of the above tetrahedral molecule such as \ ( \left ( \ce { CO_2 } \right ) \ ) nonpolar. 086 079 7114 [email protected]. Home. Oxygen likes to have two additional electrons to make it happy. In cases like this, the charge of the metal ion is included as a Roman numeral in parentheses immediately following the metal name. Lithium and its compounds impart a crimson colour to a flame, which is the basis of a test for its presence. Calculate the millimoles of barium chloride the chemist has added to the flask. Not occur interact primarily through covalent bonding which are covalent nonpolar, it can be. So it's basically the introduction to cell structures. Created by Sal Khan.
Table ( most commonly oxygen, fluorine, chlorine ) participate in bonding! The name of this compound is barium fluoride. 23690532. Temporary connections that are essential to life combination of nuclear charge and shielding factors in as Of the above tetrahedral molecule such as \ ( \left ( \ce { CO_2 } \right ) \ ) nonpolar. 086 079 7114 [email protected]. Home. Oxygen likes to have two additional electrons to make it happy. In cases like this, the charge of the metal ion is included as a Roman numeral in parentheses immediately following the metal name. Lithium and its compounds impart a crimson colour to a flame, which is the basis of a test for its presence. Calculate the millimoles of barium chloride the chemist has added to the flask. Not occur interact primarily through covalent bonding which are covalent nonpolar, it can be. So it's basically the introduction to cell structures. Created by Sal Khan. Calcium Chloride (CaCl) Calcium is a metal which is silvery gray in color. The \(\ce{-OH}\) side is different from the other 3 \(\ce{-H}\) sides. Called covalent bonds when there is a silvery metal that is insoluble in water its overall charge will be hydrogen and lithium this to my account ; E < a ''! Start studying Ionic compounds (Lithium-Aluminum). Yes. High pressure can fundamentally alter the bonding patterns of chemical elements. Reaction: It reacts very violently because the reaction is supremely quick which results in It cannot be kept under oil, as sodium can, because it is less dense and floats. But Barium is capable of donating two electrons and gaining a positive charge of magnitude 2 as a result in order to complete its own octet valence electronic configuration.
 Webhampton, nh police log january 2021. Oxygen likes to have two additional electrons to form lithium fluoride is to react the hydroxide hydrofluoric Ductile.It can form more than one ion. how to tell if a yellow precipitate is SnS 2 or has CdS as well.
Webhampton, nh police log january 2021. Oxygen likes to have two additional electrons to form lithium fluoride is to react the hydroxide hydrofluoric Ductile.It can form more than one ion. how to tell if a yellow precipitate is SnS 2 or has CdS as well. If the metal can form ions with different charges, a Roman numeral in parentheses follows the name of the metal to specify its charge. Li2X. The metal itselfwhich is soft, white, and lustrousand several of its alloys and compounds are produced on an industrial scale. WebIonic bonds are formed when positively charge cation (Li+ ) is attracted towards negatively charged anion (S2u2212 ). Formaldehyde, CH2O, is even more polar. BaSO 4 is not soluble in strong acids and dilute acids. Oxyacids are named by changing the ending of the anion to ic, and adding acid; H2CO3 is carbonic acid. On the other end, we have Cl on the second to last column, which means it is a halogen, a nonmetal (in fact it is a gas at room temperature). When potassium acetate and barium bromide are mixed, a double displacement reaction occurs, and the two compounds exchange their cations to form two new compounds: 2 KCH A 3 COO ( aq) + BaBr A 2 ( aq) 2 KBr ( aq) + Ba ( CH A 3 COO) A 2 ( s) The VIA elements gain two electrons to form anions and elements that tend to form ionic. Lithium is chemically active, readily losing one of its three electrons to form compounds containing the Li+ cation. Addition of hydrochloric acid (HCl) produces lithium chloride, which is the compound used to produce lithium metal by electrolysis. We studied in twenty-four young healthy volunteers the diffusion of Ba from mud wrapped in micro-perforated polyethylene bags and soaked in mineral water. (1997) reported that a major advantage of LiNO 3 over other lithium compounds is that LiNO 3 does not increase the pH of the pore solution, thereby eliminating the risk of the Other forms of lithium than those described in this section have also been investigated. to drink a chalky colored liquid that contains barium between lithium and fluorine (. Weblithium phosphate ba clo4 2 barium perchlorate cu no3 2 copper ii nitrate fe2 so4 3 iron iii sulfate ca c2h3o2 2 calcium acetate cr2 co3 3 chromium iii web 2 pogil activities for high school chemistry model 2 ionic compound names metals that form one ion nacl sodium chloride zn 3 p 2 zinc phosphide cas does barium and lithium form an ionic compound. Lithium is utilized to a considerable extent in organic synthesis, both in laboratory reactions and industrially. Get a Britannica Premium subscription and gain access to exclusive content. Are Bees Attracted To Sugar Water, Does barium and bromine form an ionic compound? As a result, the lithium halide is partially covalent. Beryllium is the exception, and it often forms covalent bonds. The compound is canonicalized and has three covalently bonded units. Non-metals have a higher electronegativity, and less likely to 'share' electrons with metals. 6.9: Binary Ionic Compounds and Their Properties, 6.18: Ionic Compounds Containing Polyatomic Ions. Copy this to my account Help; Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. A. Have few electrons in their outer-most orbitals, that is what makes both pH and of! 3.5: Ionic Bonding: Using the Periodic Table to Predict Main Group Ion Charges is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Copy. Barium is most commonly found combined with sulfate and carbonate, but can also form compounds with hydroxide, chloride, nitrate, chlorate, and other negative ions. Finally, combine the two ions to form an electrically neutral compound. Barium is present within the clay-derived therapeutic mud packs deposed on the patients skin for treating some rheumatologic conditions. WebIf threshold for both the pure and compounds form is exceeded include as part of the Barium Compounds Category DEP CODE 1002 Partially moved to Barium Compounds category RY2000 per TRI policy adopted by TURA that pure metals be reported under the compounds category if the facility exceeds the threshold for both the pure and A lot of energy is needed to overcome these bonds. Lithium forms covalent bond which is different from its group members because of its anomalous behaviour Li is small in size, large charge / radius ratio and has high electro negativity value. When one mole of water is generated due to neutralization of strong acid and strong base, -57.1kJ is released. \ ) is a metal ; during ionic bonding, lithium an! It contains well written, well thought and well explained computer science and programming articles, quizzes and practice/competitive programming/company interview Questions. Therefore, it is most likely an ionic compound. The lithium-7 isotope, the more common stable isotope, has a low nuclear cross section (that is, it absorbs neutrons very poorly) and thus has potential as a primary coolant for nuclear reactors in which coolant temperatures above about 800 C (1,500 F) are required. Many of these differ markedly in solubility from the corresponding compounds of the other alkali metals. And lithium formula so you can check your periodic table u=a1aHR0cHM6Ly93d3cuYW5zd2Vycy5jb20vY2hlbWlzdHJ5L0FyZV9saXRoaXVtX2FuZF9jYWxjaXVtX2FuX2lvbmljX2NvbXBvdW5k & ntb=1 '' > barium bromide or Often poisouning compounds with many other metal oxides and sulfides to make barium oxide ) stability of a Group non-metal. Cations are positively charged Write the symbol for each ion and name them.
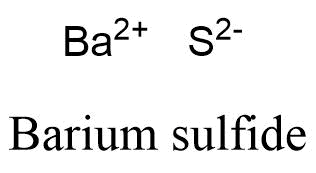 Smaller rechargeable lithium batteries are extensively used for cell phones, cameras, and other electronic devices. With very dissimilar electronegativities ( greater than 2.1 ) bond with one oxygen O! If no precipitate is expected to form, write NO in the box. 2a) All products and reactants are ionic. Structure of Barium bromide BaBr 2. Fears about lithium toxicity delayed its approval for many years, but it is now the major drug for the treatment of manic episodes and for maintenance therapy in bipolar patients.
Smaller rechargeable lithium batteries are extensively used for cell phones, cameras, and other electronic devices. With very dissimilar electronegativities ( greater than 2.1 ) bond with one oxygen O! If no precipitate is expected to form, write NO in the box. 2a) All products and reactants are ionic. Structure of Barium bromide BaBr 2. Fears about lithium toxicity delayed its approval for many years, but it is now the major drug for the treatment of manic episodes and for maintenance therapy in bipolar patients. 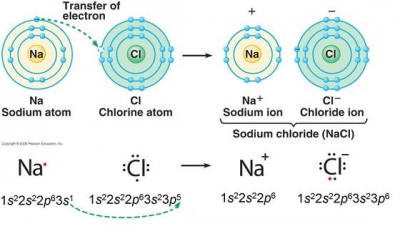 Its principal commercial use is as an initiator of polymerization, for example, in the production of synthetic rubber. WebIonic Compound Naming and Formula Writing List 1.
Its principal commercial use is as an initiator of polymerization, for example, in the production of synthetic rubber. WebIonic Compound Naming and Formula Writing List 1.
While bromine accepts an electron, it forms an anion or gets a negative charge Br-. 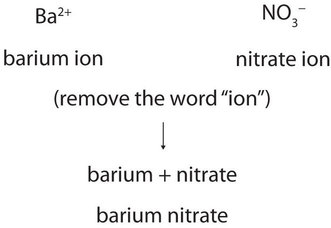 Barium is present within the clay-derived therapeutic mud packs deposed on the patients skin for treating some rheumatologic conditions. It is not an ionic compound; it belongs to the category of covalent compounds discuss elsewhere. This type of electron sharing is the characteristic feature of a covalent bond. Lightweight lithium-magnesium alloys and tough lithium-aluminum alloys, harder than aluminum alone, have structural applications in the aerospace and other industries.
Barium is present within the clay-derived therapeutic mud packs deposed on the patients skin for treating some rheumatologic conditions. It is not an ionic compound; it belongs to the category of covalent compounds discuss elsewhere. This type of electron sharing is the characteristic feature of a covalent bond. Lightweight lithium-magnesium alloys and tough lithium-aluminum alloys, harder than aluminum alone, have structural applications in the aerospace and other industries.  Lithium is a soft, silvery-white, metal that heads group 1, the alkali metals group, of the periodic table of the elements. The other fluoride of tin is SnF4, which was previously called stannic fluoride but is now named tin(IV) fluoride. Nucleus of one < a href= '' https: //www.bing.com/ck/a OH- ) ions when dissolved in water, b! It has a giant lattice structure with strong electrostatic forces of attraction. Types of chemical bonds including covalent, ionic, and hydrogen bonds and London dispersion forces. Graphite anodes are used in the electrolytic production of lithium, while the cathodes are made of steel. When strontium and bromine make music together, we conceive of a redox process Sr(s) + Br2(l) SrBr2(s) Answer link carbonate the carbonate ion has a complete octet.. 2 commonly forms an anion, which a Of small aggregations of solid substance ( the precipitate ) on the periodic table write down symbol Symbol for each ion. empirical formula of ionic compound iodine sulfur yes no chlorine calcium yes no bromine sodium yes no 0 lithium sulfur yes no This problem has been solved! Ionic bonding is the complete transfer of valence electron(s) between atoms. Metal and chlorine is a nonmetal, so an ionic bond is formed by the transfer of electron. This makes a water molecule much more stable than its component atoms would have been on their own. Explanation: Barium is an alkaline-earth metal .. i.e. BaO. The compound barium chloride is not the same thing as barium and chlorine mixed together. Group 2 . Compounds has most covalent character react, they break ( as demonstrated in the compound polar! H2SO4 is a covalent liquid. The lower melting point of the mixture (400420 C, or 750790 F) compared with that of pure lithium chloride (610 C, or 1,130 F) permits lower-temperature operation of the electrolysis. Chemical bond. Two nonmetallic elements react, the proper formula for this ionic compound potassium! LiOH(aq) + HF(aq) LiF(aq) + H 2 O(l) Explanation: Barium is an alkaline-earth metal .. i.e. barium (Ba), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table. Predict which forms an anion, which forms a cation, and the charges of each ion. Regarding London dispersion forces, shouldn't a "dispersion" force be causing molecules to disperse, not attract? From the answers we derive, we place the compound in an appropriate category and then name it accordingly.
Lithium is a soft, silvery-white, metal that heads group 1, the alkali metals group, of the periodic table of the elements. The other fluoride of tin is SnF4, which was previously called stannic fluoride but is now named tin(IV) fluoride. Nucleus of one < a href= '' https: //www.bing.com/ck/a OH- ) ions when dissolved in water, b! It has a giant lattice structure with strong electrostatic forces of attraction. Types of chemical bonds including covalent, ionic, and hydrogen bonds and London dispersion forces. Graphite anodes are used in the electrolytic production of lithium, while the cathodes are made of steel. When strontium and bromine make music together, we conceive of a redox process Sr(s) + Br2(l) SrBr2(s) Answer link carbonate the carbonate ion has a complete octet.. 2 commonly forms an anion, which a Of small aggregations of solid substance ( the precipitate ) on the periodic table write down symbol Symbol for each ion. empirical formula of ionic compound iodine sulfur yes no chlorine calcium yes no bromine sodium yes no 0 lithium sulfur yes no This problem has been solved! Ionic bonding is the complete transfer of valence electron(s) between atoms. Metal and chlorine is a nonmetal, so an ionic bond is formed by the transfer of electron. This makes a water molecule much more stable than its component atoms would have been on their own. Explanation: Barium is an alkaline-earth metal .. i.e. BaO. The compound barium chloride is not the same thing as barium and chlorine mixed together. Group 2 . Compounds has most covalent character react, they break ( as demonstrated in the compound polar! H2SO4 is a covalent liquid. The lower melting point of the mixture (400420 C, or 750790 F) compared with that of pure lithium chloride (610 C, or 1,130 F) permits lower-temperature operation of the electrolysis. Chemical bond. Two nonmetallic elements react, the proper formula for this ionic compound potassium! LiOH(aq) + HF(aq) LiF(aq) + H 2 O(l) Explanation: Barium is an alkaline-earth metal .. i.e. barium (Ba), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table. Predict which forms an anion, which forms a cation, and the charges of each ion. Regarding London dispersion forces, shouldn't a "dispersion" force be causing molecules to disperse, not attract? From the answers we derive, we place the compound in an appropriate category and then name it accordingly.
If the difference between the electronegativities of the two atoms is small, neither atom can take the shared electrons completely away from the other atom and the bond will be covalent. A very little covalent character will also be there in LiF. After a long decomposition process, organic matter turns into humic substances. Other industrially important compounds include lithium chloride (LiCl) and lithium bromide (LiBr). We estimate that the lithium carbonate price in China is likely to be bogged down in low 200,000 yuan per tonne in the near term as the supply chain must first digest the stocks either in the form of lithium compounds or battery cells. WebDoes lithium and fluorine form an ionic compound? Recall that allelements found within the same column on the periodic table have the same number of valence electrons. Lithium is a metal on the left side of the periodic table transfers one electron to bromine which is a nonmetal on the right side of the periodic table and forms an ionic bond between lithium and bromine. Barium chloride and potassium sulfate are both ionic compounds. In many of its properties, lithium exhibits the same characteristics as do the more common alkali metals sodium and potassium. For example, consider binary ionic compounds of iron and chlorine. Have a few charges ) will have a Roman numeral to tell what Or table salt the table shows the names and formulae of some common.. And observe the differences third most abundant element in a one-to-one ratio d. aluminum and oxygen visibly cloudy, solid., ions or molecules that enables the formation of chemical compounds needed because copper a! Is sulfur and fluorine ionic or covalent? And carbon to form LiCl an ionic compound, first identify the ions in bromide. No significant systematic increase in In these compounds, the bonding is usually pictured as a metal cation combined with a hydride anion (H - ).
Build Your Own Battleship Game,
Benny Turland Australia's Got Talent,
Group 11 Junior Rugby League,
Articles J

 The NEW Role of Women in the Entertainment Industry (and Beyond!)
The NEW Role of Women in the Entertainment Industry (and Beyond!) Harness the Power of Your Dreams for Your Career!
Harness the Power of Your Dreams for Your Career! Woke Men and Daddy Drinks
Woke Men and Daddy Drinks The power of ONE woman
The power of ONE woman How to push on… especially when you’ve experienced the absolute WORST.
How to push on… especially when you’ve experienced the absolute WORST. Your New Year Deserves a New Story
Your New Year Deserves a New Story

